Reportable range seems like a very simple study. Something we all have to do in laboratories. But, there’s still quite a bit of confusion on how to interpret results. – Sten Westgard, Director of Client Services and Technology at Westgard QC.
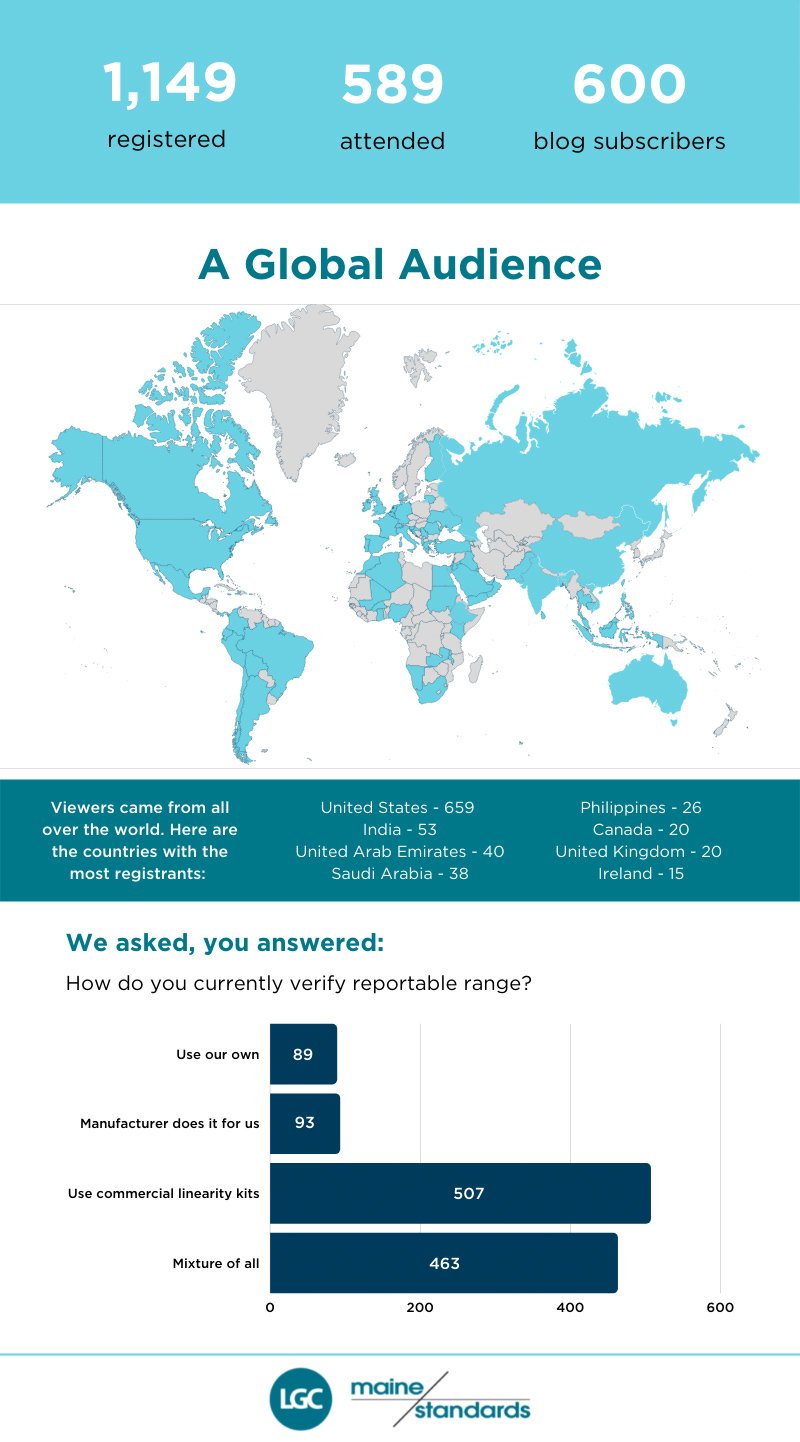
It’s clear laboratory professionals are looking to eliminate that confusion. More than 1,000 registered for Sten Westgard’s reportable range and linearity webinar. Sponsored by LGC Maine Standards, the presentation packed plenty of information, examples and advice into one hour.
The why, when and what of testing
Sten reviewed CLIA regulations, focusing on the many situations that trigger testing. He shared three goals for reportable range studies: confirm the range listed in the product insert is reasonably correct, confirm you can use the test to get results for patients, and check the box for regulatory compliance.
One of the challenges he discussed is that some CLIA and CLSI EP06 requirements don’t provide the necessary details, leaving laboratories to find the information elsewhere. Allowable deviation from linearity? Allowable bias? Total allowable errors? Several sources are available to get the specifications you need, including CLIA, CAP, Australia’s RCPA, Germany’s Riliback, and the Westgard QC website.
Gaining insight into what is being measured and how to obtain results in the right range makes managing your laboratory easier. As Sten told participants, “It’s not enough for us to know what the right experiment is. We need to understand how to do it correctly. Implement correctly. Interpret correctly.”
And the how
In creating a reportable range study it’s important to know where it fits within your validation plan. The webinar addressed the difference between validation and verification of linearity. Sten also tackled questions about upper and lower limits and non-linearity.
When it comes to analyzing the data, Sten explained plotting observed values or the means of multiple measurements for each standard instead of using the “assigned value” as well as calculating regression statistics for a line that represents the linear part of the range.
Since CLIA and CAP mandate verifying linearity every six months and after specific events such as major maintenance, relocation or disruption, reportable range is a constant. Be sure you have the right information to have confidence in your plan.
Toeing the Line: The Right Way to Verify Your Reportable Range and Linearity available on demand. Earn 1-hour P.A.C.E credit
Earn 1-hour P.A.C.E credit
Webinar Statistics